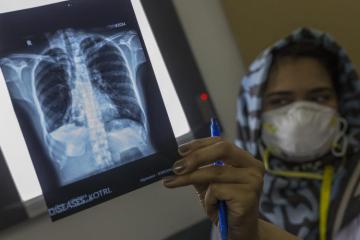
endTB aims to find shorter, less toxic and more effective treatments for ‘multidrug-resistant TB’ (MDR-TB) through:
- access to new drugs
- two clinical trials
- advocacy at national and global levels
Covering 18 countries, the project is a partnership between Partners In Health, Médecins Sans Frontières, Interactive Research & Development and financial partners Unitaid and the Transformational Investment Capacity (TIC) of MSF.
You can contact us, or follow us on Twitter.