Nurses prepare patients’ treatments in the pharmacy of the ambulatory ward at the National Centre for Tuberculosis and Lung Disease in Georgia’s capital, Tbilisi. (Daro Sulakauri/MSF)
Testing novel, short, all-oral regimens for MDR-TB
Médecins Sans Frontières (MSF), Partners In Health (PIH), Interactive Research and Development (IRD), and their partners have launched two major clinical trials which seek to revolutionize treatment for the toughest strains of tuberculosis (TB), the world’s leading infectious disease killer.
The two clinical trials are sponsored by MSF. The Principal Investigators are Carole Mitnick (Harvard Medical School) and Lorenzo Guglielmetti (MSF).
Epicentre, Harvard Medical School, the Institute of Tropical Medicine Antwerp and UCSF are partners of the trials.
Why these clinical trials?
Conventional treatments for MDR-TB are long (up to 24 months), ineffective (only 59% treatment success in 2018) and often cause terrible side effects, including acute psychosis and permanent deafness. All the while, patients ingest up to 14,000 pills and some of them have to endure months of painful, daily injections. Moreover, the costliness, difficulty and length of current treatments make them hard to implement in many high-burden countries.
To address the problem, the two clinical trials use the first TB drugs developed in almost 50 years — bedaquiline and delamanid — to find radically shorter (6 or 9 months), more tolerable, injection-free treatments for MDR-TB.
1. endTB trial (fluoroquinolone-susceptible MDR-TB)
The endTB clinical trial enrolled 754 patients across eight countries: Georgia, India, Kazakhstan, Lesotho, Pakistan, Peru and South Africa. These are all countries with significant TB burdens, where MSF, PIH or IRD support local MDR-TB treatment activities.
Study design
endTB was a randomized, controlled, Phase III clinical trial testing five new, all oral, 9-month regimens compared to the current standard of care. Randomization was outcome-adapted, rather than fixed. This means that the probability of being randomized to regimens changed as the outcomes were reported: more patients were assigned to regimens that were producing better outcomes. A summary of the study protocol has been published and is publicly available here.
Study treatment regimens
Each experimental regimen contains at least one new drug, in combination with up to four companion drugs. The control regimen is the local standard of care consistent with latest WHO recommendations, including bedaquiline and/or delamanid if indicated.
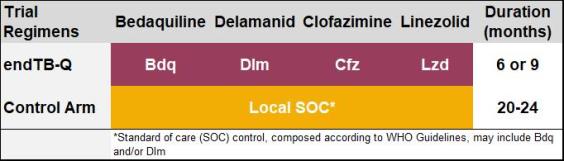
2. endTB-Q trial (fluoroquinolone-resistant MDR-TB)
The endTB-Q clinical trial enrolled 323 patients across six countries (India, Kazakhstan, Lesotho, Pakistan, Peru, and Vietnam) to find simpler, less toxic, shorter regimen for fluoroquinolone-resistant MDR-TB.
Study design
This is a randomized, controlled, Phase III clinical trial testing a new, all oral regimen compared to the current standard of care.
Study treatment regimen
The experimental regimen contains bedaquiline, delamanid, clofazimine, and linezolid, and is delivered for 24 or 39 weeks. endTB-Q adopts a personalized medicine approach, by individualizing the treatment duration based on baseline characteristics and treatment response of each participant.
The control arm regimen designed and delivered according to local standard of care and consistent with latest WHO guidelines and may include bedaquiline and/or delamanid in addition to other drugs.
Study oversight
Several independent groups provide formal external oversight of the study protocols, implementation, operation and analysis:
- Institutional Review Boards or Ethics Committees at MSF, Harvard Medical School, IRD, Antwerp Institute of Tropical Medicine, and in each country participating in the clinical trial
- Independent Scientific Advisory Committee (SAC)
- Global Community Advisory Board, convened by the Treatment Action Group
- Data Safety and Monitoring Board (DSMB)
These groups will continue to assure the relevance of the trials for clinical care and policy, their safety for participants, and that global standards for clinical research are met or exceeded.
Technical resources
- For endTB reference documents, such as the summary of the study protocol, please see our Resources section.
- View the endTB page on ClinicalTrials.gov
- View the endTB-Q page on ClinicalTrials.gov.